Lithium (Li) metal has been recognized as the promising anode material for rechargeable batteries because of its high theoretical capacity of 3860 mAh g−1 and the lowest electrode potential (−3.04 V v.s. the standard hydrogen electrode). However, Li metal usually forms a fragile solid electrolyte interphase (SEI), which is not sufficiently dense to passivate the surface of Li metal. During stripping and plating, the SEI cannot suppress the side reaction between Li metal and electrolyte. The poor SEI may also induce the heterogeneous deposition of Li, leading to the formation of notorious dendrites. The uncontrolled Li dendrites not only consume cyclable Li but also accumulate irreversible “dead lithium” owing to the loss of electric connection. Coulombic efficiency (CE) is then dramatically reduced. At a fixed loading, the deliverable capacity decays rapidly. In addition, Li dendrites may cause safety issues such as short circuits and catastrophic cell failures, which hinder the practical application of Li metal batteries.
Recently, Li Aidong’ group (College of Engineering and Applied Sciences of Nanjing University) proposed to use the molecular layer deposition technology (MLD) to induce the formation of LiF-rich interphase for lithium metal anodes.
This work reports a zincone (ZnHQ) MLD technology and its application for inducing LiF-rich SEI on a copper nanowire (CuNW) anode. Zincone is a zinc-based hydroquinone (HQ) where Zn replaces hydrogen of HQ. As shown in Figure 1a, the hydroxyl (-OH) modified Cu was grafted with HQ during the MLD. Diethylzinc further reacts with the hydroxyl groups of grafted HQ to yield ZnHQ. HQ is selected as the backbone molecule because it is relatively stable upon lithiation and a single chain of ZnHQ has a terminal oxygen, which can serve as the nucleophilic group to attack Li bistrifluoromethanesulfonimide (LiTFSI) upon negative polarization. The resultant LiF-rich SEI (Figure 1b,c) can facilitate Li ion diffusion and suppress the dendritic Li growth that was usually induced on a pristine Cu (Figure 1d,e). More importantly, zinc atoms can induce the deposition of Li metal owing to the lithiophilicity. Furthermore, the high surface area of porous scaffold and CuNWs reduces the local current density and prolongs the Sand's time. As a result, the CuNW@ZnHQ electrode demonstrates superior cyclability for over 7000 h at a capacity of 1 mAh cm−2 and can maintain more than 300 h at a high loading capacity (15 mAh cm−2). In addition, CuNW@ZnHQ was paired with NCM523 at a capacity of 3.2 mAh cm−2 and show excellent cyclability with 90% capacity retention for 1000 cycles. This work provides an alternative approach to developing nanoscale interfacial coatings for Li metal and demonstrates that the zincone MLD strategy may serve as a potential technology for next-generation high-energy Li metal anodes.
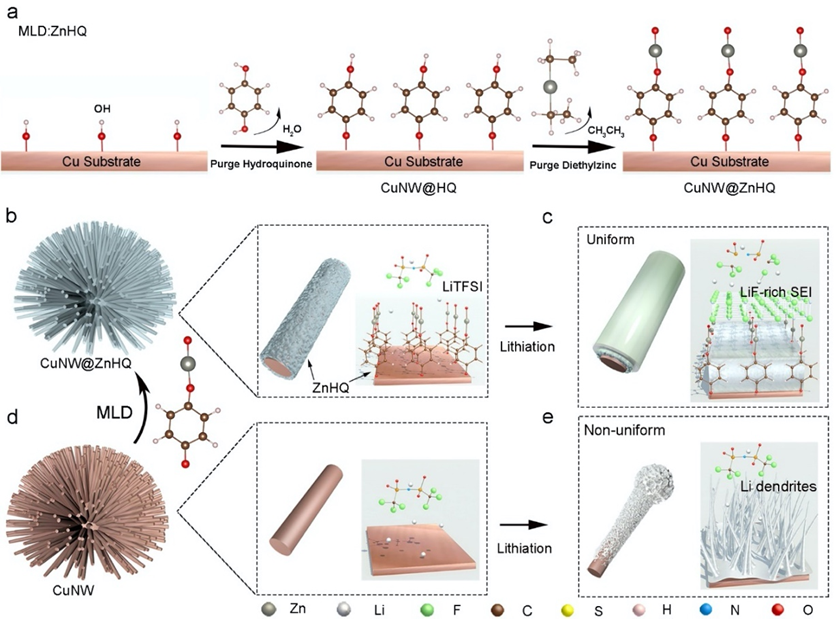
Figure 1 Schematic illustration of a) the chemical reactions in zincone MLD; b) SEI formation on CuNW@ZnHQ and c) Uniform Li deposition process under ZnHQ-induced Li-F rich SEI; d) SEI formation on CuNW and e) Non-uniform Li deposition process on CuNW.
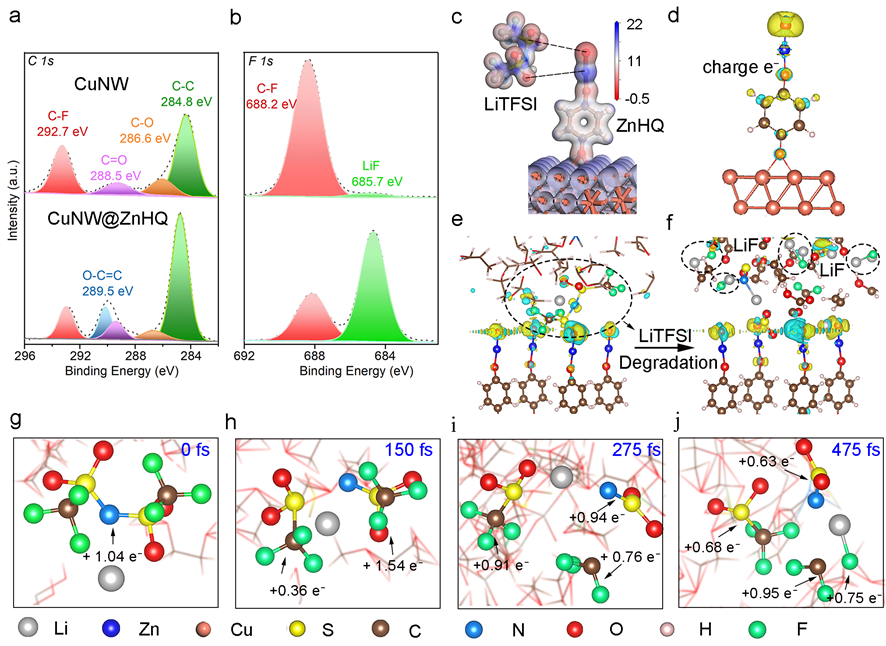
Figure 2 Mechanism analysis of the LiTFSI degradation: XPS spectra of CuNW and CuNW@ZnHQ: a) C 1s and b) F 1s signals. c) Electrostatic potential distribution of LiTFSI and zincone at the isosurface of electron density, d) Electron difference diagram of a negatively charged ZnHQ on Cu. Electron difference diagrams of e) before and f) after LiTFSI decomposition. Snapshots of AIMD simulations to show the degradation dynamics: g) 0 fs, h) 150 fs, i) 275 fs, and j) 475 fs.
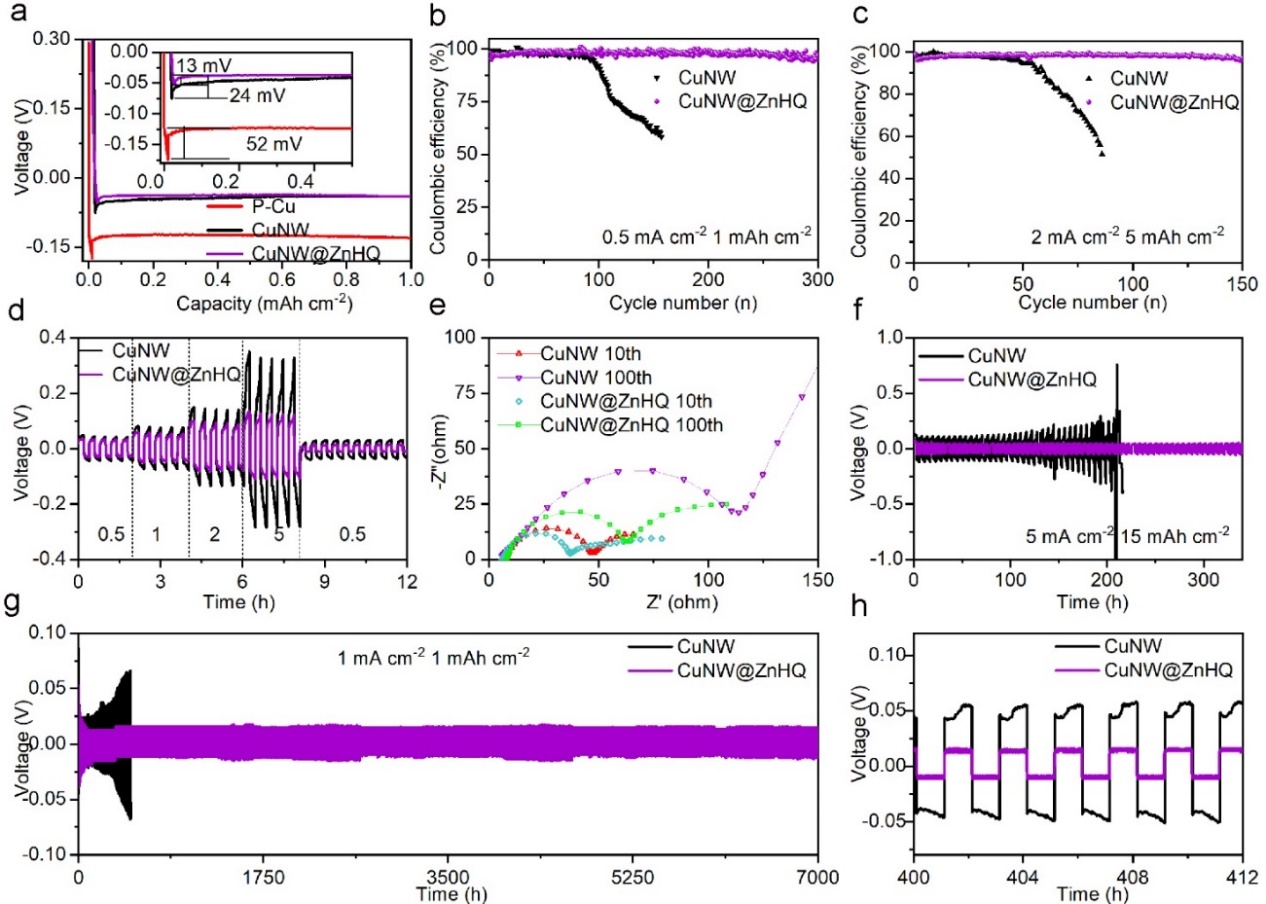
Figure 3 Electrochemical properties and cycling stability of P-Cu, CuNW@ZnHQ and CuNW. a) Voltage curves of Li plating at a capacity of 1 mAh cm−2 and a current density of 0.5 mA cm−2. CEs of Li plating/stripping b) at 0.5 mA cm−2 (1 mAh cm−2) and c) at 2 mA cm−2 (5 mAh cm−2) with a cut-off voltage of 1.0 V vs. Li+/Li during stripping. d) Rate capability and voltage curves under various current densities from 0.5 to 5 mA cm−2. e) EIS spectra of CuNW and CuNW@ZnHQ in the 10th and 100th cycle. Time-voltage profiles under density of at f) 5 mA cm−2 (15 mAh cm−2) and g, h) 1 mA cm−2 (1 mAh cm−2).
Shaozhong Chang, Jiabin Fang, Kai Liu, Zihan Shen, Lin Zhu, Xin Jin, Xuejin Zhang, Chaoquan Hu, Huigang Zhang, Ai-dong Li. Molecular-Layer-Deposited Zincone Films Induce the Formation of LiF-Rich Interphase for Lithium Metal Anodes. Adv. Energy Mater. 2023. 2204002.
https://onlinelibrary.wiley.com/doi/abs/10.1002/aenm.202204002。
This work was financially supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, and the Jiangsu Province. Zhang Xuejin professor has given strong help in COMSOL simulation. At the same time, it has also been supported by National Laboratory of Solid State Microstructures, Collaborative Innovation Center of Advanced Microstructures, and College of Engineering and Applied Sciences, Nanjing University, Jiangsu 210093, China.

