Binuclear Cu complex catalysis enabling Li–CO 2 battery with a high discharge voltage above 3.0 V
Carbon capture and utilization (CCU) is receiving increasing attention in the fields of CO2 reduction, global warming mitigation, and potential Mars exploration. Various CCU technologies that can convert CO2 into value-added chemicals, such as methane dry reforming, hydrogenation, electrochemical reduction, and photocatalytic reduction, have been developed. In the past decade, a kind of energy storage device of Li–CO2 battery was proposed, providing an attractive strategy to utilize CO2 and generate electrical energy of high specific energy. However, the practical output voltage of Li–CO2 battery is often below 2.5 V, sometimes even lower than 2.0 V in previous reports. On the one hand, the common discharge product Li2CO3 is insulating and insoluble. On the other hand, electrochemical decomposition of Li2CO3 usually triggers a large number of side reactions, which further decreases the energy efficiency and cycling stability of the battery. Although the currently reported solid catalysts can reduce the charging overpotential to some extent, they have minimal effect in enhancing the discharge voltage. In order to increase the output voltage of Li–CO2 battery and meet the requirements of high-quality electrical energy, Prof. Ping He and Prof. Haoshen Zhou in College of Engineering and Applied Sciences, Nanjing University propose a soluble binuclear copper(I) complex (Cu(I) RM) as liquid catalyst in Li–CO2 battery for the first time. Based on the exploration of catalytic mechanism and the regulation of discharge/charge reaction path, the work increases the output voltage of Li–CO2 battery to more than 3.0 V.
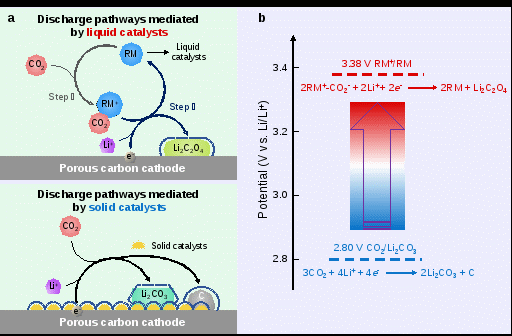
Figure 1 Schematic comparison of solid catalysts-mediated and liquid catalysts-mediated discharge reactions in Li–CO2 battery.
Specifically, the Li–CO2 battery with Cu(I) RM exhibits a remarkably promoted electromotive voltage up to 3.38 V. Electrochemical and spectroscopic investigations show that the reaction route of Li–CO2 battery involving Cu(I) RM is as follows: Firstly, Cu(I) RM captures CO2 to form a bridged Cu(II)-oxalate adduct; Secondly, the adduct is reduced with the formation of initial Cu(I) RM and Li2C2O4 products during discharge; Finally, Li2C2O4 discharge products are decomposed in the charge process. With the addition of Cu(I) RM, the Li–CO2 battery delivers an enhanced discharge plateau of about 3.04 V, an enlarged discharge capacity of 5846mAh g−1, and a reduced charge voltage plateau of around 4.27 V. Besides, the Li–CO2 battery exhibits steady cyclability over 80 cycles. To further reduce the charging polarization, Ru nanoparticles deposited on carbon cathode are incorporated as the cathode catalyst in this Cu(I) RM-involved Li–CO2 battery. Benefiting from the synergistic catalytic effect of Cu(I) RM and Ru catalysts, a similar discharge voltage of 3.01 V, a promoted discharge capacity of 8058mAh g−1, and a remarkably decreased charge voltage of about 3.99 V are achieved. Furthermore, the battery shows robust cycle stability over 400 cycles. This study raises the output voltage of Li–CO2 battery to more than 3.0 V and realizes a Li2CO3-free discharge pathway, thereby improving the electrochemical performance of Li–CO2 battery and propelling the practical application.
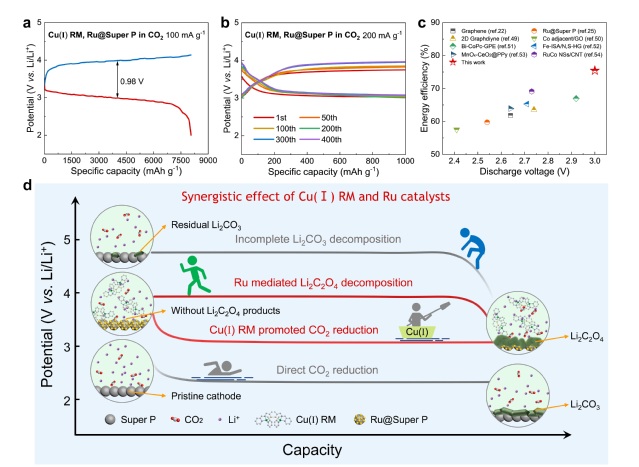
Figure 2 Synergistic catalytic effect of Cu(I) RM liquid catalysts and Ru@Super P solid catalysts in Li–CO2 battery.
The work titled "Binuclear Cu complex catalysis enabling Li–CO2 battery with a high discharge voltage above 3.0 V" was published online on Feb. 1st, 2023 in Nature Communications (https://doi.org/10.1038/s41467-023-36276-8). Prof. Ping He and Prof. Haoshen Zhou in College of Engineering and Applied Sciences, Nanjing University are the corresponding authors of the paper, and Ph.D. candidate Xinyi Sun enrolled in 2019 and post-doctoral Xiaowei Mu in College of Engineering and Applied Sciences, Nanjing University are the co-first authors of the paper. The research work was also supported and assisted by Prof. Cheng-Hui Li's group in School of Chemistry and Chemical Engineering, Nanjing University. This research was funded by the National Natural Science Foundation of China, the National Key R&D Program of China, the Natural Science Foundation of Jiangsu Province of China, Key R&D Project funded by Department of Science and Technology of Jiangsu Province, Science and Technology innovation fund for emission peak and carbon neutrality of Jiangsu province, and China Postdoctoral Science Foundation. This work was also supported by National Laboratory of Solid State Microstructures, State Key Laboratory of Coordination Chemistry, Collaborative Innovation Center of Advanced Microstructures, Jiangsu Key Laboratory of Artificial Functional Materials, and Center of Energy Storage Materials & Technology in Nanjing University.

