Acute gastroenteritis, a common inflammatory intestinal disease, is usually caused by food-borne Salmonellae, such as Salmonella enterica serovarTyphimurium(S. Typhimurium). Antibiotic therapeutics to combat intestinal pathogen infections often exacerbate microbiota dysbiosis and impair mucosal barrier functions. Probiotics are promising strategies because they inhibit pathogen colonization and improve intestinal microbiota imbalance. Nevertheless, their limited targeting ability and susceptibility to oxidative stress have hindered their therapeutic potential. To tackle these challenges, Ces3 is synthesized by in situ growth of CeO2 nanozymes with positive charges on probiotic spores, facilitating electrostatic interactions with negatively charged pathogens and possessing a high reactive oxygen species (ROS) scavenging activity. Importantly, Ces3 can resist the harsh environment of gastrointestinal tract. In mice with S. Typhimurium-infected acute gastroenteritis, Ces3 shows potent anti-S. Typhimurium activity, thereby alleviating S. Typhimurium dissemination into other organs. Additionally, owing to its O2 deprivation capacity, Ces3 promotes the proliferation of anaerobic probiotics, reshaping a healthy intestinal microbiota. This work demonstrates the promise of combining antibacterial, anti-inflammatory, and O2 content regulation properties for acute gastroenteritis therapy.
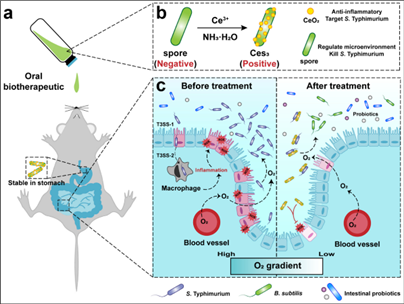
Schematic illustrating acute gastroenteritis microenvironment and Ces3-enabled microenvironmental regulation for targeted acute gastroenteritis therapy.
Journal reference:
Gen Wei, Wanling Liu, Yihong Zhang, Zijun Zhou, Yuting Wang, Xiaoyu Wang, Shuaishuai Zhu, Tong Li, Hui Wei, Nanozyme-Enhanced Probiotic Spores Regulate the Intestinal Microenvironment for Targeted Acute Gastroenteritis Therapy. Nano Letters 2024, 24 (7), 2289-2298.

