Professor Zhaosheng Li and his team: Sustainable and effective electrolysis of seawater for hydrogen production using renewable energy
Professor Zhaosheng Li and his team from the College of Engineering and Applied Sciences achieved new advancements in seawater splitting for hydrogen production and published a research article titled “Ultrastable electrocatalytic seawater splitting at ampere-level current density” (Nature Sustainability, 2024, 7, 158‒167).
The electrolysis of seawater using renewable energy sources is expected to be a low-cost technology for producing green hydrogen. However, the high concentration of Cl− in seawater poses a challenge for producing hydrogen from seawater electrolysis. One of these challenges is the competition between oxygen evolution (OER) and Cl− oxidation. Another more serious challenge is electrocatalyst corrosion or deactivation of Cl− through surface adsorption. Therefore, it is necessary to develop electrocatalysts with Cl− corrosion resistance and high selectivity for the OER.
To address these challenges, Li's group designed an electrocatalyst based on layered double hydroxyl compounds (CoFe-LDHs). LDHs have a unique layered structure, large specific surface area, adjustable composition and high OER activity in alkaline environments, and they are expected to be used as OER electrocatalysts for electrolyzing alkaline seawater. However, Cl− intercalation in seawater can lead to structural collapse and inactivation. Carbonate ions were inserted within the LDH layers to maintain structural stability (Fig. 1a). CO32−, which has a high charge, small ion size and triangular plane configuration, will exhibit strong electrostatic interactions with the positively charged main layer. Therefore, CoFe-LDHs containing CO32− (CoFe-Ci) usually have narrower layers and more stable structures than CoFe-LDHs containing other interlayer anions (such as interlayer NO3−, CoFe-Ni). In the process of seawater electrolysis, the anion exchange of CO32− and Cl− between the layers of the CoFe-Ci nanosheets is almost absent, which inhibits the collapse or spalling of the LDH layered structures. This assumption is consistent with the order of LDH ion exchange equilibrium constants (CO32−> SO42− > OH− > F− > Cl− > Br− > NO3−). Moreover, the high negative charge density of CO32− endows the CoFe-Ci nanosheets with good repulsive properties for Cl− between layers. In addition, CO32− helps stabilize high-priced active metal ions in the main layer, improving catalytic performance. The team also anchored graphene quantum dots (GQDs) to the surface of CoFe-Ci (Fig. 1a). Similar to interlayer CO32−, negatively charged GQDs prevent adverse Cl− adsorption or coordination at the active site on the surface of CoFe-Ci nanosheets.
Time-of-flight secondary ion mass spectrometry (TOF-SIMS) confirmed that both CO32− and GQDs have repulsive effects on Cl−. The Cl species content of the electrocatalyst follows the order CoFe-Ci@GQDs < CoFe-Ci ≪ CoFe-Ni. X-ray photoelectron spectroscopy (XPS) analysis suggested that the electron-donating effects of CO32− and GQDs may contribute to the stabilization of high-energy intermediate states at the active site on the surface. The abundant oxygen-containing functional groups in GQDs enable them to act as proton regulators, facilitating the hydrolytic dissociation and proton-coupled electron transfer processes of the OER.
CoFe-Ci@GQDs electrocatalyst electrolyzes seawater for hydrogen production at a current density of ~1.25 A cm−2 for 2800 hours (Fig. 1b). The electrocatalyst was used as a catalyst for oxygen and hydrogen production, and a photovoltaic seawater electrolysis device was built (Fig. 1c). The OER selectivity was close to 100%, and the conversion efficiency of solar energy to hydrogen energy reached 18.1% at a large current of ~440 mA. This study signifies a significant advancement in producing hydrogen through electrolyzing seawater using renewable energy sources.
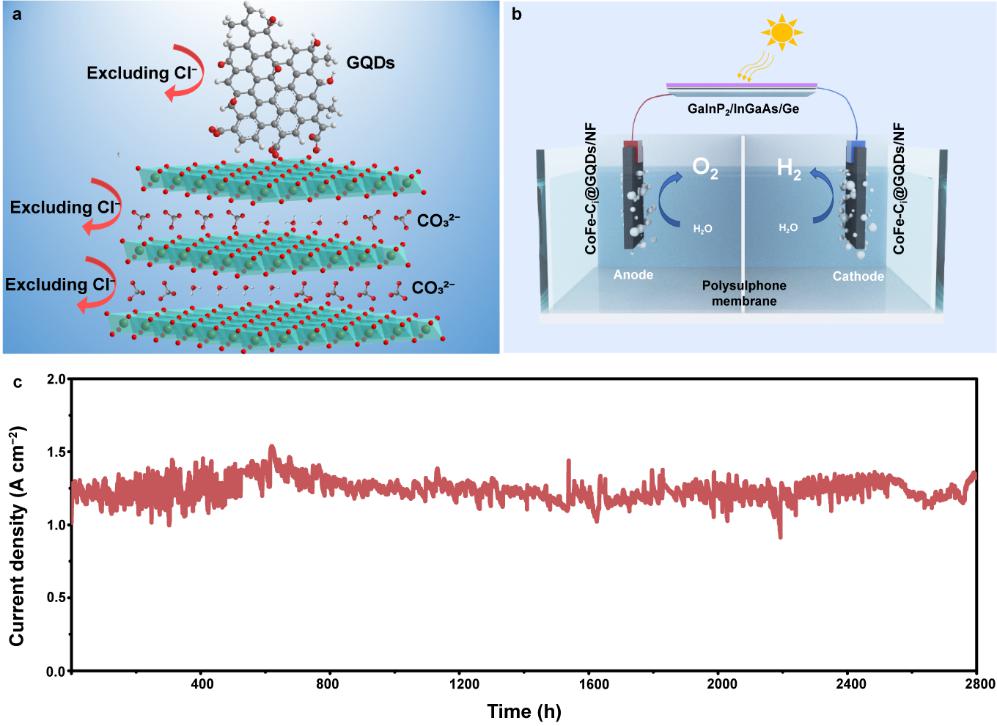
Figure 1. a, CoFe-LDHs for CO32− intercalation and surface modification with GQDs (CoFe-Ci@GQDs). b, Stability test of CoFe-Ci@GQDs/NF in alkaline simulated seawater (1 M KOH + 0.5 M NaCl). c, Schematic diagram of a photovoltaic seawater electrolysis device based on CoFe-Ci@GQDs/NF.

