Coverage enhancement accelerates acidic CO2 electrolysis at ampere-level current with high energy and carbon efficiencies
Acidic CO2 electroreduction (CO2R) using renewable electricity holds promise for high-efficiency generation of storable liquid chemicals with up to 100% CO2 utilization. However, the strong parasitic hydrogen evolution reaction (HER) limits its selectivity and energy efficiency (EE), especially at ampere-level current densities. Here Miao Zhong's group at the College of Engineering and Applied Sciences at Nanjing University presents that enhancing CO2R intermediate coverage on catalysts promotes CO2R and concurrently suppresses HER. They identified and engineered robust Cu6Sn5 catalysts with strong *OCHO affinity and weak *H binding, achieving 91% Faradaic efficiency (FE) for formic acid (FA) production at 1.2 cm−2 and pH 1. Notably, the single-pass carbon efficiency reaches a new benchmark of 77.4% at 0.5 cm−2 over 300 hours. In situ electrochemical Fourier-transform infrared spectroscopy revealed Cu6Sn5 enhances *OCHO coverage ~2.8× compared to Sn at pH 1. Using a cation-free, solid-state-electrolyte-based membrane-electrode-assembly (SSE-MEA), 0.36 M pure FA at 88% FE was produced over 130 hours with a marked full-cell EE of 37%.
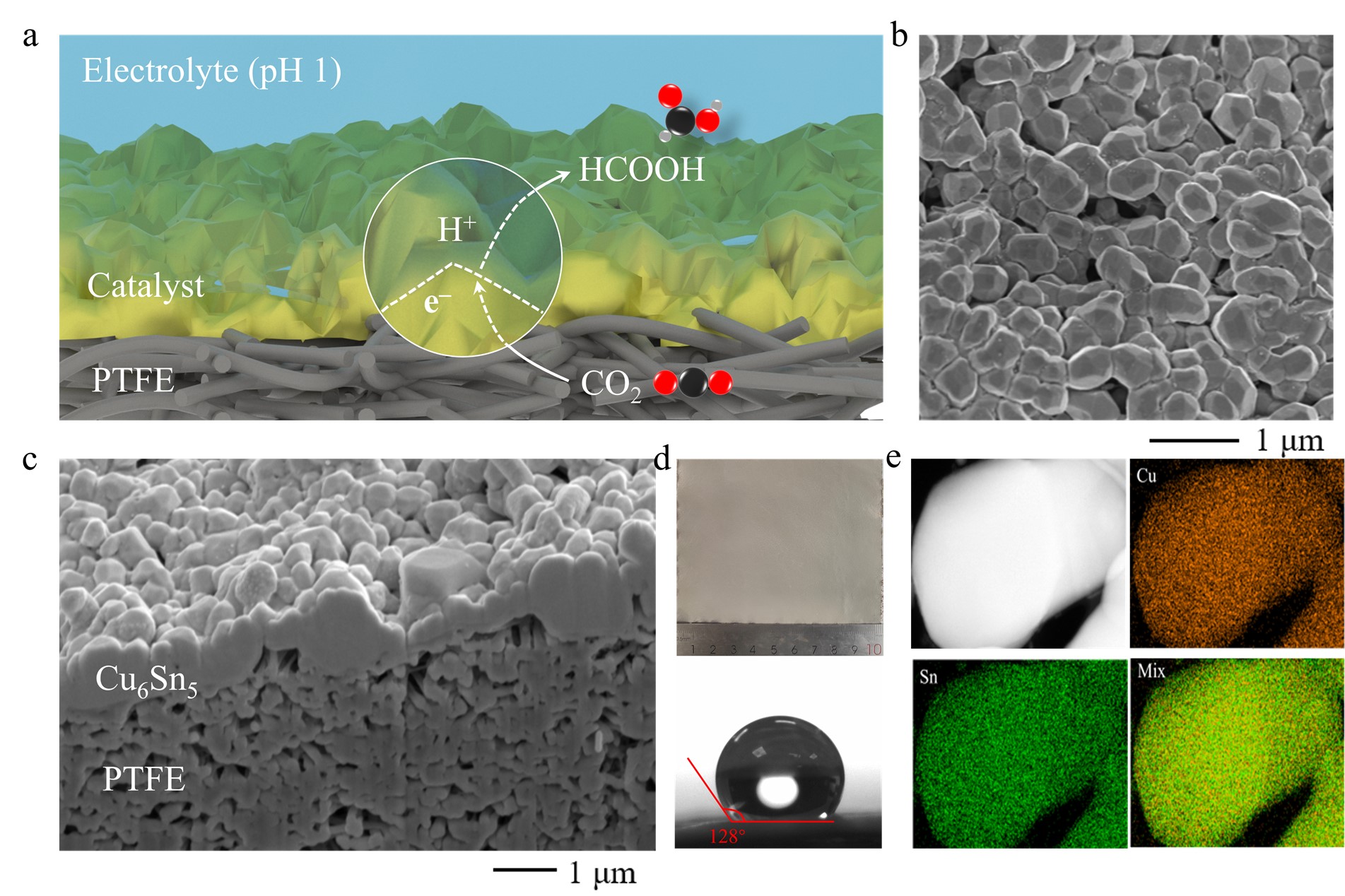
Figure 1 Characterization of Cu6Sn5 catalysts on a gas diffusion electrode.
Fig.1a shows a schematic of the CO2R process on Cu6Sn5/PTFE electrodes. The top-view and cross-sectional scanning electron microscope (SEM) images in Fig. 1b and 1c show the 300–600 nm Cu6Sn5 nano-to-micro particles densely packed on the PTFE substrate. This Cu6Sn5-on-PTFE structure was synthesized over a large scale via thermal evaporation (Fig. 1d), exhibiting hydrophobicity, as indicated by a water contact angle of 128°. This hydrophobicity enables CO2 diffusion through the gaps between the particles on the PTFE sides to the catalyst surface (Fig. 1a). Energy dispersive X-ray spectroscopy in transmission electron microscopy (STEM-EDX) analysis revealed a uniform distribution of Sn and Cu over the majority of the Cu6Sn5 particles (Fig. 1e).
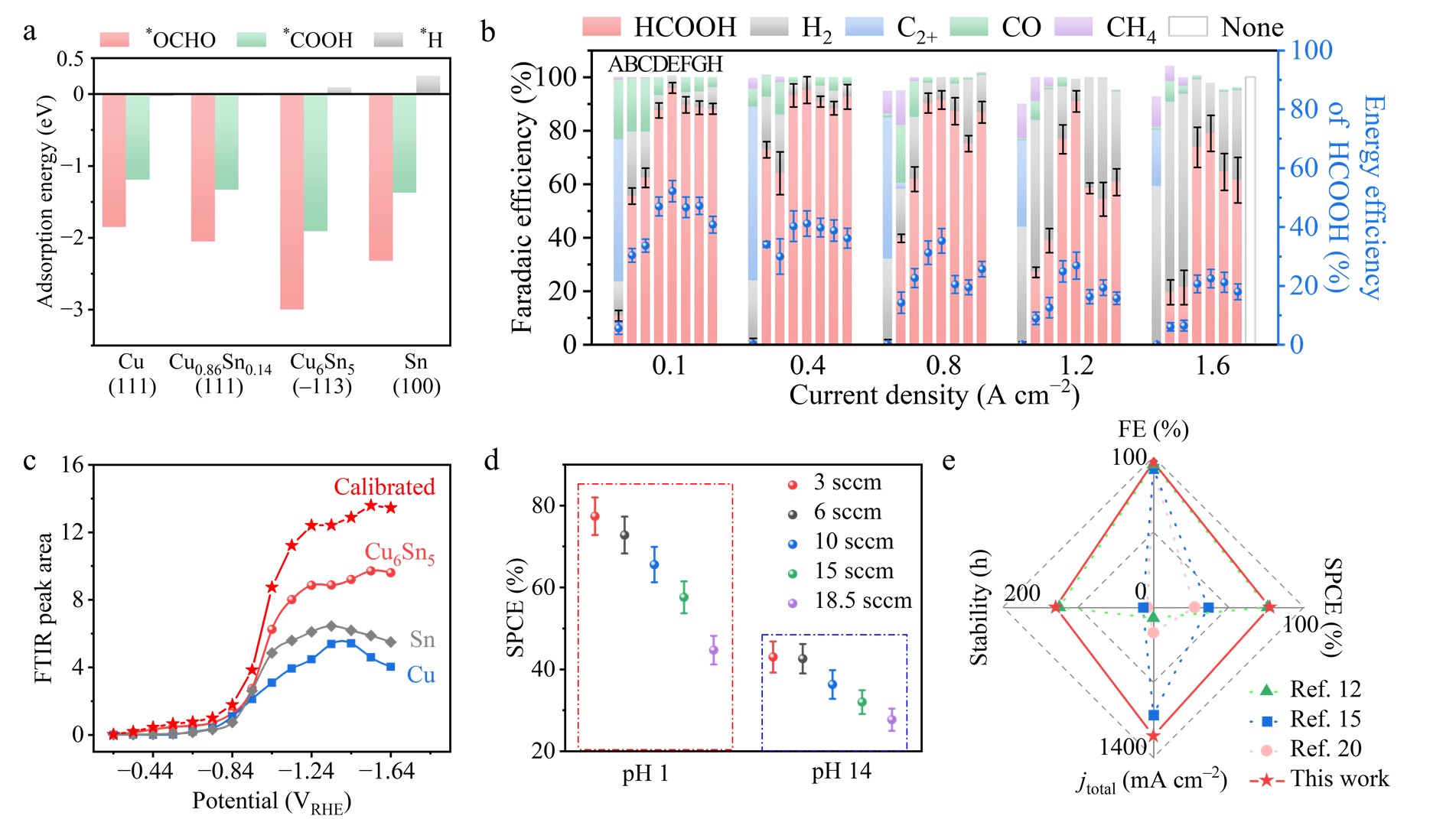
Figure 2 Electrochemical performance of CO2 reduction with Cu6Sn5 catalyst in strong acid (pH 1) electrolytes.
In a 3 M KCl and 0.05 M H2SO4 electrolyte at pH 1, Cu6Sn5 exhibited the highest selectivity, reaching up to 96% at −1.4 VRHE for CO2R to formic acid in Fig. 2b. Remarkably, Cu6Sn5 showed a high FE of above 90% for FA production across a wide range of current densities from 0.4 to 1.2 A cm−2 (Fig. 2b). We examined the SPCE for Cu6Sn5 under a constant current density of 0.5 A cm−2 in a flow cell equipped with a 1.7 × 1.7 cm2 serpentine channel reaction area at various CO2 flow rates and electrolyte pH levels. Fig.2d presents a maximum SPCE of 77.4%, achieved at a CO2 flow rate of 3 standard cubic centimeters per minute (sccm) under pH 1 conditions. According to DFT calculations, the selectivity for FA across Cu (111), Cu0.86Sn0.14 (111), Cu6Sn5 (−113), and Sn (100) surfaces exhibits a volcano plot (Fig. 2a), with Cu6Sn5 (−113) displaying the highest energy difference between the two pivotal intermediates, favoring the adsorption of *OCHO over CO. Notably, adhering the established calibration relationship, in situ electrochemical attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy shows the plateau *OCHO peak intensity on Cu6Sn5 measured at potentials above −1.04 VRHE in the CO2R electrolyte is ~2.8 times higher than that of Sn, with all measurements conducted under identical conditions (Fig. 2c). These results point toward an enhanced surface coverage of *OCHO on Cu6Sn5, which facilitates selective FA production during acidic CO2R. We compared this work with previous reports on the CO2R-to-FA production under acidic conditions in terms of elevated current density, FE, SPCE, and stability in Fig. 2e.
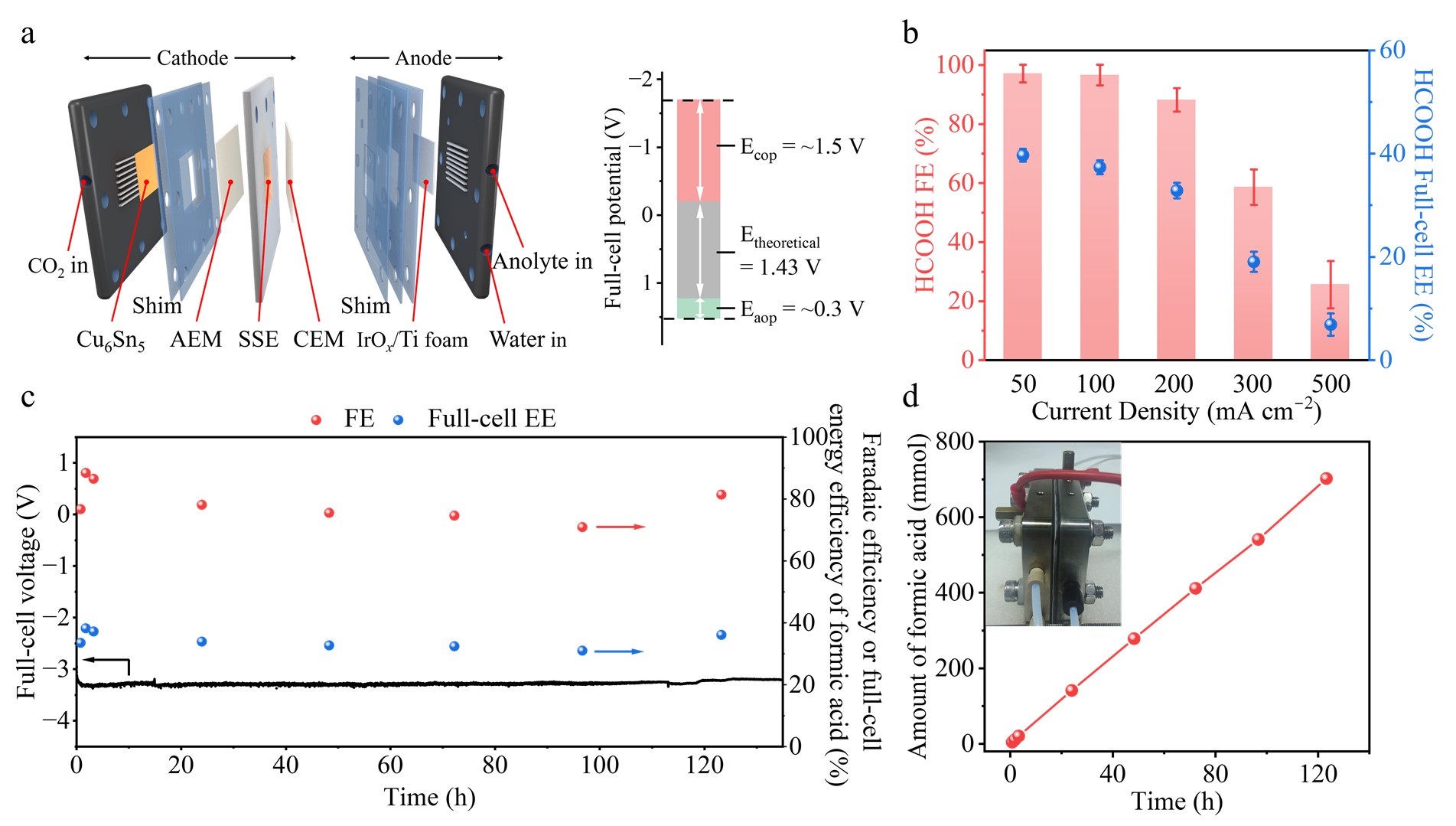
Figure 3 Electrochemical CO2R performance with Cu6Sn5 catalyst using a cation-free, solid-state-electrolyte-based membrane electrode assembly (SSE-MEA) electrolyzer.
To produce a pure FA solution, we conducted CO2R using a cation-free, SSE-MEA electrolyzer (Fig. 3). Using the Cu6Sn5 catalyst, the FA FE reached ~96% at 100 mA cm−2. The full-cell voltage was −3.7 V and the full-cell energy efficiency was over 37%. We quantified the amount of FA produced using IC, NMR, and pH measurements. The results confirmed the production of 2.6 liters of 0.36 M pure FA solution with a production rate of 20 mL h−1 over a continuous 130-h CO2R process.
The above results were published as a research article online (Nat. Commun. 2024, 15, 1711).Miao Zhong from the College of Engineering and Applied Sciences, Nanjing University, and Fanglin Che from the University of Massachusetts Lowell are the co-corresponding authors.Xiaohan Yu (College of Engineering and Applied Sciences, Nanjing University), Yuting Xu (University of Massachusetts Lowell), and Le Li (College of Engineering and Applied Sciences, Nanjing University) are the co-first authors. The authors thank the National Natural Science Foundation of China, the National Key R&D Program of China, the Program for Innovative Talents and Entrepreneurs in Jiangsu, etc for the funding support.
DOI: 10.1038/s41467-024-45988-4

