The gas-open cell architecture has always been the restriction of the development of lithium-oxide(air) battery. On the other hand, the high specific capacity and energy provides by oxygen related to anionic redox is the cheese many reluctant to give up. The paper developed sealed battery based on oxygen redox reaction from the perspective of control and utilization and realized the highly reversible battery with long life. Recently, Haoshen Zhou’s research group of our department made new progress in lithium-oxide(air) battery. The manuscript titled as A high-energy-density and long-life lithium-ion battery via reversible oxide-peroxide conversion is published on Nature Catalysis(DOI:doi.org/10.1038/s41929-019-0362-z). Prof. Haoshen Zhou is the corresponding author.

As a matter of fact, back when he was still in Advanced Industrial Science and Technology (AIST) in 2010, Prof. Haoshen Zhou led his research group to develop sealed lithium-oxide battery. Numerous attempts and endeavor were made by several researchers including Dr. Yonggang Wang (now a professor of Fudan University), Dr. Huiqiao Li (now a professor of The Huazhong University of Science and Technology) , Dr. Ping He (now a professor of Nanjing University), Dr. Haijun Wei (now a professor of Beijing University of Technology). After difficult exploration and many obstacles, the focus was concentrated on the effective utilization of interconversion between Li2O/ Li2O2 as well as the prevention of irreversible products (LiO2, O2-,O2 and so on). Therefore, the priority of research was to keep the redox process related to oxygen within where Li2O/ Li2O2 could reversibly transform and to avoid gaseous oxygen and superoxo production. The goal was to realize stable and highly reversible long-term battery cycling based on redox reactions of oxygen.
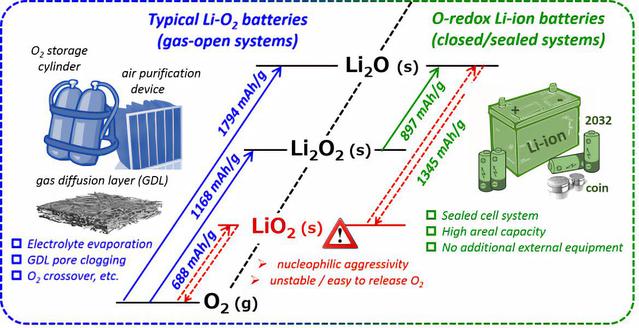
Figure 1 From open to closed, schematic representations of oxygen-based beyond-intercalation Li battery systems.
The open system lithium-oxygen battery needs to carry the cumbersome weight of accessories such as cylinders, air filters, etc. At the same time, the diffusion of gas is easily affected by the electrode structure and solid discharge products. Whilethe sealed lithium-oxygen system can achieve a very high energy density (compared to existing cathode materials of lithium battery). In the meanwhile, lithium superoxide is considered to be in a very nucleophilic aggressive form. Therefore, when designing the research, we plan to avoid the formation of superoxide as much as possible, abandon the three-electron ( two oxygen atoms) capacity that Li2O/ LiO2 may provide, and instead conservatively ensure the reversible Li2O/ Li2O2-basedtwo-electron ( two oxygen atoms) reaction to the largest extent. Between capacity expansion and reversibility, effectively guaranteeing reversible capacity is the key to success. The thermodynamic theoretical potential is as follows:
O2+2Li++2e-= Li2O2 ; E0= 2.96 V vs. Li/Li+ ; (1168 mAh/g~Li2O2)
O2 + 4Li++4e-= 2Li2O; E0= 2.91 V vs. Li/Li+ ; (1794 mAh/g~Li2O)
Li2O2+2Li++2e-= 2Li2O;E0= 2.86V vs. Li/Li+ ;(897 mAh/g~Li2O )

Figure 2 Characterization of original anode. rGO carrying Ir as catalytic matrix, mixed with Li2O as anode.
In the classic lithium-oxygen battery system, the contact of solid catalyst and discharge product (Li2O2) has always been the fundamental issue that troubles the design of electrodes and materials. We usedmetal Ir with a strong catalytic effect as the catalyst, which is supported on a soft reduced graphene oxide( rGO) substrate, and tiny (nanoscale) Li2O particles are evenly mixed into the Ir-rGO matrix. In this way, the catalyst, active material and conductive matrix are tightly combined.

Figure 3 Charge curves during first cycle, related in situ spectra and quantitative analysis
According to the calculations in Fig. 1, the capacity will reach 897 mAh / g (converted to the mass of the entire electrode, including Li2O + Ir + rGO + PVdF binder, specific capacity is 570 mAh / g ~ cathode) when the oxidation from Li2O to Li2O2 is fully achieved. If the charge capacity is mechanically kept at the theoretical conversion capacity, the potential will gradually increase at the end of charging.
It is worth noting that, especially after the charge capacity exceeds 450mAh / g ~ cathode, the irreversible capacity loss increases when the charge depth extends, that is, the reversibility of the entire electrochemical cycle deteriorates when the charge depth extends. The detailed data collected by insitu electrochemical Raman and gas chromatography-massspectrometry show that when the charge capacity is smaller than 420mAh/ g, the Li2O loaded on the positive electrode is gradually converted into a single peroxide product, which is Li2O2. After the positive electrode capacity exceeds 450mAh/ g, superoxides (including superoxide radical ions and LiO2 adsorbed on the surface) and oxygen (O2) are gradually released. It is precisely due to the irreversible products produced during these deep charging processes, that leads to the inevitable irreversible capacity loss during discharge.
In other words, to suppress irreversible products and improve the reversibility of electrode reactions, we must make a compromise between capacity expansion and stability. More importantly, through characterizations of different aspects, we successfully determined the warning line capacity between reversibility and irreversibility (~ 420 mAh / g ~ cathode), which provides valuable data forlater effective utilization of isochoric cycle with reversible capacity.

Figure 4 Proposed reaction mechanism. Mechanism of the interaction between metal catalyst Ir and Ir-rGO matrix.
From the CV curve it could be seen that when potential is above 3.0V vs. Li / Li +, there is obvious oxygen release. Release of oxygen after overcharge meets the theoretical thermodynamic OER potential threshold. Meanwhile, after controlling the charging voltage, CV shows a set of symmetrical redox peaks. Combined with the information obtained in Fig. 3, control of both voltage andcapacity are equally important and indispensable. The rGO electrode without metal Ir (Fig. 4b), the initial charging voltage exceeds the OER warning line. The charging voltage being kept at a high level inevitably leads to continuous oxygen release during the charging process.
It is worth noting that the EIS spectrum during the charging process shows that with metal Ir catalyst, Rct exhibits a certain increase, and the Warburg impedance obviously changes from capacitive reactance to impedance. While in the absence of Ir catalyst, except for the apparent increase in Rs, there is no significant change in Rct and Warburg impedance. The informationEIS sends us: with the Ir metal catalyst, the outer surface layer of Li2O particles is gradually converted into Li2O2 with poorer electronic conductivity; in the absence of Ir, the gap caused by the oxygen evolution decomposition of Li2O makes the battery contact resistance increase. The core of the problem focuses on how the metal Ir suppresses the newly formed Li2O2 shell from OER decomposition, so that the Li2O in the core is continuously converted into Li2 O2? XPS analysis shows that the metal Ir does not keep the chemical / electronic state intact. Instead trace oxidation occurs at the initial stage of charging, forming a stable inter-metallic state.
At the same time, the newly formed Li2O2 shell gradually releases Li, forming an extremely unstable Li-deficient peroxide Li2-xO2. After the combination of those two, due to covalent effect, a stable Ir-Li2-xO2 protective shell (Angew. Chem. Int. Ed., 2019, doi.org/10.1002/ange.201901350) is formed to protect the newly formed Li2O2 from OER oxygen evolution decomposition. It is undeniable that the stability of the Ir-Li2-xO2 protective shell is relative. As the thickness of the Li2O2 shell gradually accumulates (when the charge depth increases), Rct gradually rises, and the subsequent rise of electrochemical polarization and potential will eventually break through the protective layer.
All in all, the factors influencing the trend of overpotential rise are different: with Ir, the electrochemical polarization comes from the increase in the electronic conductivity caused by the accumulation and thickening of the Li2O2 shell; without Ir, the high charge potential and the extremely strong polarization are attributed to the spatial separation between the active material particles and the conductive substrate caused by the decomposition of Li2O. It is of vital importance to form Ir-Li2-xO2 protective shell.

Figure 5 Schematic of effective control of charge capacity. Spectroscopic and quantitative proof of the reversible transformation of Li2O / Li2O2 during the cycle.
Controlling the charge capacity within a safe rangefavors reversible conversion of Li2O/ Li2O2 during the cycle. Based on the parameter determined in Fig. 3, we set the red line for safeoxygen-free and superoxo-free charging for actual use as 400mAh/ g ~ cathode (up to 70% of the conversion capacity of Li2O). The comprehensive information collected by insitu Raman, titration analysis and insitu gas chromatography-mass spectrometry shows that the redox reaction circulated under the cutoff capacity of 400 mAh / g is completely based on the reversible transformation of Li2O / Li2O2. The idea of effective control of the charge capacity within the safe range is also used for LiCoO2 (the conversion capacity of LiCoO2 is only 50%), a classic commercial lithium-ion battery cathode material.

Figure 6 Performance of battery based on reversible electrochemical conversion positive electrode reaction of Li2O / Li2O2 .
The chargecutoff capacity is fixed at 400 mAh / g ~ cathode (specific capacity calculation is based on the mass of the overall positive electrode load, including: Li2O active material, metal Ir catalyst, rGO substrate, PVDF binder), half-cell can maintain up to 2000 highly reversible cycles (99.5% stable coulombic efficiency). Based on the the total cathode material load, the energy density is 1090Wh / kg, which has obvious advantages compared with the traditional lithium-ion battery cathode materials (Fig. 6c). It is often misunderstood that, under the premise of ensuring a large enough capacity, the low voltage platform is not a disadvantage for energy density, but a advantage which stabilize oxygen behavior and related electrolyte systems. In the meanwhile, as shown in Fig. 6d, compared with the conventional lithium-oxygenbatteries and sealed lithium-oxygen batteries that have been reported, the results after comprehensive analysis (energy efficiency, surface capacity, cycle stability) show obvious advantages of this system. In addition, the full battery assembled with Si negative electrode can also achieve stable reversible long cycle and considerable energy density output.
In this work, sealed lithium-oxygen batteries based on the reversible conversion of Li2O/ Li2O2 was successfully achieved. Conceptually, this work creatively reveals the importance of controlling and effectively utilizing reversible Li2O/ Li2O2 conversion. In terms of characterization, insitu / exsitu spectroscopy combined with accurate quantitative analysis was used to calibrate the reversible redox reaction region and prove the reversible conversion of the reaction products. In mechanism, the role and formation principle of metal Ir and its related stable protective layer are clarified. In terms of performance, we have successfully achieved high specific energy (1090 Wh / kg, calculated based on the mass of all positive loads), high reversibility (stable coulombic efficiency ~ 99.5%), high energy efficiency (overpotential only ~ 0.12 V), and high stability (2000 cycles or more) closed system lithium-oxygen battery cycle.
(Source: WeChat OfficialAccount "Rational Science")

