Distant metastasis of cancer cells is the most common cause of death for cancer patients. Among them, the liver is the ideal organ for cancer metastasis due to its rich blood supply. Surgical resection is currently the only radical treatment, but it only works for patients with a single metastatic lesion and has strict limits on the size of the lesion. Patients who meet the above conditions often account for only a small part of patients with liver metastasis. Most patients have already formed several to tens of metastases (diffuse liver metastases) in the liver when they are diagnosed, which makes it impossible to realize radical removal. For those patients, only palliative treatment based on chemotherapy can be used. The survival benefitsaredampened and the patients have to suffer severe liver function damage. How to selectively remove metastases lesions and maximize the protection of liver function has become a research topic of great clinical significance. In recent years, nanotargeting drugs have brought dawn to the treatment of metastatic cancer. However, problems such as loss of tumor cell biomarkers or structural mutations during cancer metastasis have limited the practical application of nanotargeting drugs.
In response to this problem, Yong Hu’s research group of College of Engineering and Applied Science, Xiqun Jiang’s of School of Chemistry and Chemical Engineering of Nanjing University, and Zhen Gu’s of the University of California, Los Angeles cooperated internationally and proposed a marker-free liver metastasis treatment, which could effectively remove cancer cells while maximally maintaining liver cell function.
In our design, we selected mesoporous silicon as carriers for photodynamic drugs (A unit), and coupled non-stoichiometric tungsten oxide nanospheres (B unit) to the surface of the silicon spheres through the Cathepsin B enzyme-responsive amino acid segment, forming a planet-satellite structure. Its working principle is shown in Figure 1. The high enzymatic activity in tumor cells leads to the separation of A and B units, and promotes the two to bind with mitochondria and nucleus respectively. Through sequential laser irradiation, oxidative phosphorylation and glycolysis controlled by mitochondria and nucleus are inhibited, cutting off the energy source of cancer cells-ATP production. Normal liver cells and Kupffer cells lack sufficient enzyme digestion activity, and the structure of the nanocarriers is maintained. This results in that the active oxygen generated by the first round of laser irradiation is absorbed by the non-stoichiometrictungsten oxideswith a satellite distribution. In the second round of laser irradiation, the W (IV) as the active center of the B unit which acts as the photothermal generating medium is oxidized by the first round active oxygen, thus losing the light-heat conversion ability, and finally protects normal tissue from heat damage.
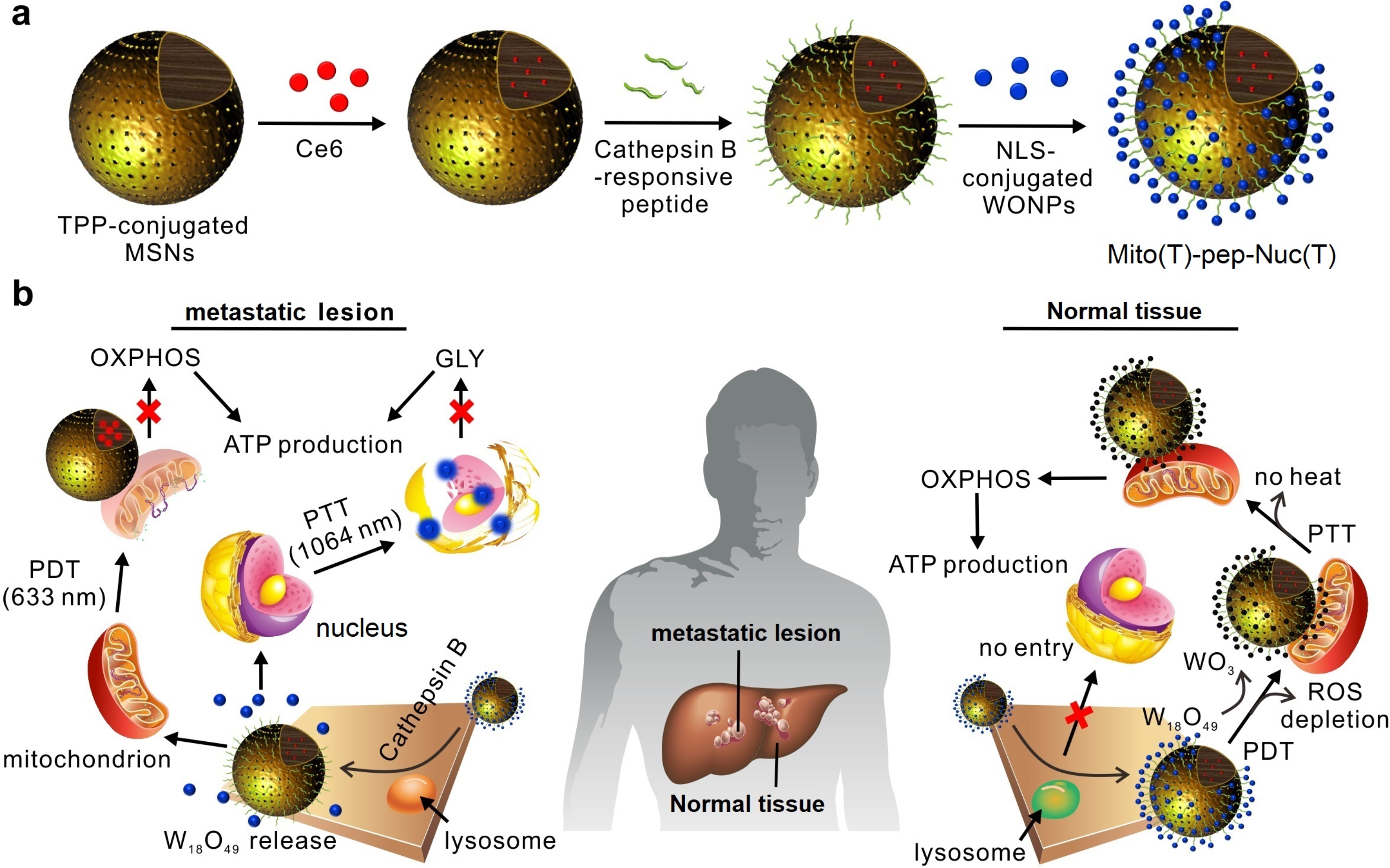
Figure 1. Schematic of the working principle of self-quenching nanocarriers.
We further explored the practical application of the mentioned self-quenching mechanism and related molecular biological mechanisms. Seahorse XF analysis results show that the material we designed can quickly deplete ATP storage in cancer cells and thereby induce programmed cell death. Based on the mining of bioinformationof genomics and proteomics data, we confirmed that the combined treatment deceived the energy receptors in cancer cells, and at the same time further accelerated the ATP depletion of cells by inducing endoplasmic reticulumand DNA damage repair (Fig. 2). Compared with the first-line advanced liver cancer targeting drug sorafenib, we confirmed that the self-quenching treatment designed in this work significantly destroyed liver metastases while preserving liver function, and ultimately obtained twice the survival benefits of chemotherapy . Based on the fact that the treatment plan we designed is independent on the effectiveness of cell markers, we believe that this treatment will hopefully play a key role in the treatment of metastasis of organ cancers other than the liver in the future as well.
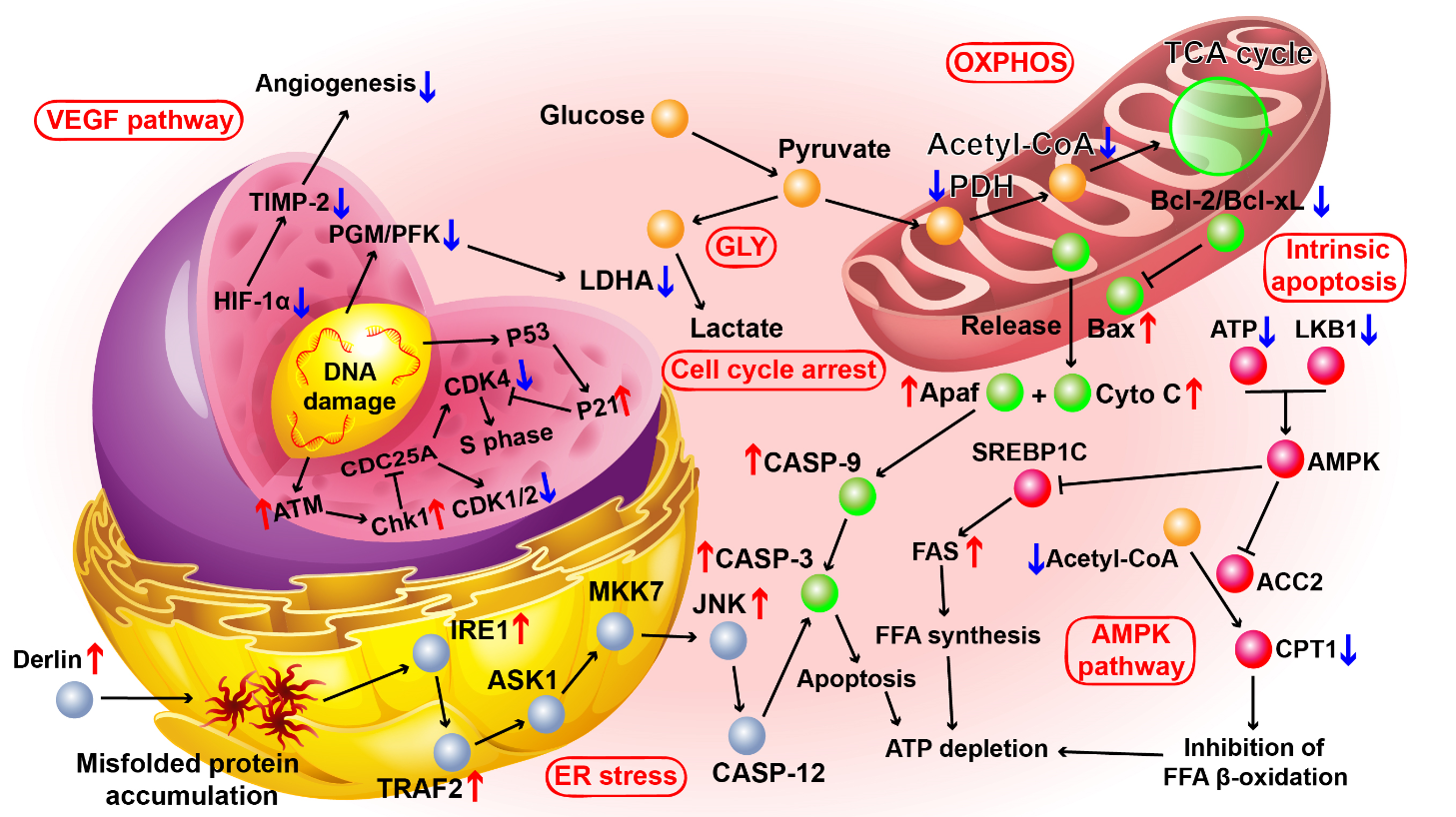
Figure 2. KEGG pathways.
The manuscript titled as Eradication of unresectable liver metastasis through induction of tumour specific energy depletion is published on Nature Communications. College of Engineering and Applied Science of Nanjing University is the first corresponding affiliation. Grade 15 PhD student Da Huo of College of Engineering and Applied Science is the first author. Prof. Yong Hu, Prof. Xiqun Jiang and Prof. Zhen Gu are the co-corresponding authors. This work also receives supported from PhD student Jianfeng Zhu, Chao Zhang, Wei Jiang, Xingyu Luo of Prof. Yong Hu’s research group, as well as Dr. Guojun Chen of the University of California, Los Angeles, and Prof. Qian Chen of Suzhou University. This research is funded by the National Key R & D Program of China, the National Natural Science Foundation of China, and the Excellent Projects Exploration Plan of Nanjing University.

