Recently, the research group of Prof. Haoshen Zhou and Associate Prof. Shaohua Guo successfully prepared the layered sodium-rich manganese-based sodium-ion battery cathode material Na1.2Mn0.4Ir0.4O2 for the first time by solid-phase sintering. The material has a typical O3 phase structure. The doping of iridium stabilizes the electrochemical properties of the material and reduces the precipitation of O2 during the charging and discharging process. Using in-situ X-ray diffraction technology, in-situ Raman technology, X-ray absorption spectroscopy and other characterization techniques combined with first-principles density functional theory calculations, the researchers have successfully revealed the phase change process and the mechanism of electrochemical reaction, providing new aspects for the development and design of sodium-ion batteries, and exploring a new system for the study of layered electrode materials. Related work has been published in Advanced Materials, entitled Manganese based Na-rich materials boost anionic redox in high-performance layered cathodes for sodium-ion batteries. (DOI: 10.1002/adma.201807770)
Sodium-ion battery has long been regarded as an important technique for the development of large-scale energy storage. In recent years, the major aim of researches on sodium-ion batteries is to develop sodium-ion battery electrode materials with high specific energy and low cost. Due to the similarity of sodium and lithium in chemistry, the research of sodium-ion batteries can draw on the valuable experience gained during the development of lithium ions batteries, which includes further increasing the charge and discharge capacity of the electrode material by changing the chemical valence of anions.
In recent studies, the redox reaction of anions in the layered structure of sodium deficiency (mainly P2 type) and sodium richness (mainly O3 type) is observed. The research on layered sodium-rich materials has been relatively slow because the existing research on sodium-rich materials consumes precious metals of 5d elements-ruthenium and iridium-as the core of the electrochemical reaction, which increases the cost. Moreover, a certain amount of O2 release in reported layered sodium-rich materials impedes the stable cycling of electrode materials.
Based on the limitations above, the research group of Prof. Haoshen Zhou and Associate Prof. Shaohua Guo dedicates to find a suitable technical route from the perspective of reducing cost and stabilizing the cycle performance of layered sodium-rich electrode materials to develop a new layered sodium-rich sodium ions electrode material.
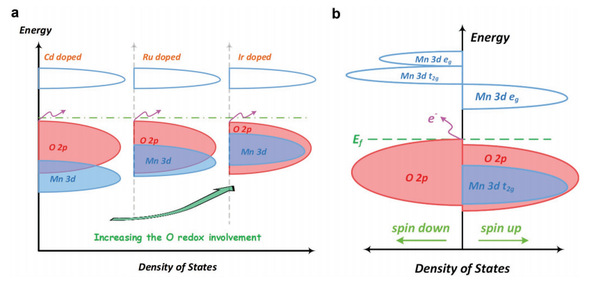
Figure 1 (a) Schematic diagram of the state density structure of the Mn-based layered sodium-rich material after Cd Ru Ir doping; b) Schematic diagram of the energy band structure of Ir-doped Na1.2Mn0.4Ir0.4O2
As shown in Figure 1, the energy band changes of various elements doped in manganese-based sodium-rich materials are studied with first-principles density functional theory calculations. By continuously improving the covalent property of the doping elements, the researchers find that the 3d state of manganese originally buried deep at the bottom of the valence band shows an upward shift, providing a new unbonded O2p empty orbit near the Fermi surface. The phenomenon suppresses the generation of O2 to some extent and plays a role in stabilizing the circulation because 5d transition metal (TM) can form a strong covalent bond of TM-O with O and subsequently inhibit the release of O2. Therefore, it is proposed to adopt iridium doping to realize the construction of layered sodium-rich manganese-based sodium ion electrode material.
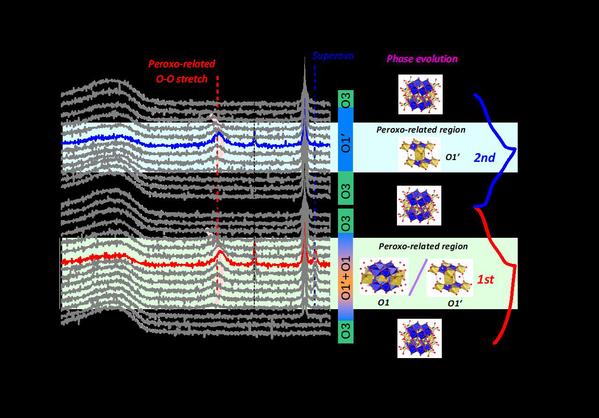
Figure 2 left) In-situ Raman detection of the O valence change in Na1.2Mn0.4Ir0.4O2 during the electrochemical process; middle) Schematic diagram of the phase transition process of Na1.2Mn0.4Ir0.4O2 during charging and discharging; right) Na1.2Mn0.4Ir0.4O2 material first two charge-discharge cycles
Furthermore, through in-situ X-ray diffraction techniques, in-situ Raman techniques, X-ray absorption spectroscopy, X-ray photoelectron spectroscopy and other characterization techniques, the researchers deeply explore the structural changes and charge compensation mechanism of Na1.2Mn0.4Ir0.4O2 material during charging and discharging. As shown in Figure 2, the study shows that at the beginning of the first charge cycle, as the sodium ions are stripped off, the layers slip and the material changes from the O3 phase to a mixture of the O1 phase and the O’1 phase. The difference between the O1 phase and the O'1 phase is the degree of sliding between the layers. O1 has a higher degree of an interlaminar slip than the O’1 phase. After charging, the material has both O1 phase and O'1 phase. When the discharging starts, the material returns to the original O3 phase. In the subsequent electrochemical process, the O1 phase disappears, and the material does not move dramatically between layers. Therefore, with the extraction and insertion of sodium, the material undergoes a phase transition from O3 to O’1 and back to O3. The in-situ Raman characterization results show that during the first charging and discharging circle, peroxygen bonds are detected, indicating the presence of O22- in the material. At the same time, superoxide bonds are also detected, which closely relates to the decomposition of the electrolyte used in the research process. The in-situ gas mass spectrometry GC-MS study finds that significant O2 release is not detected during charging and discharging, but CO2 release is detected. This phenomenon can be explained with the decomposition of the electrolyte. The superoxide bond disappears during the second cycle, leaving only the reversible peroxide type O22-.
By X-ray absorption spectroscopy and X-ray photoelectron spectroscopy characterization of the original, charged and discharged electrode sheets, a significant price change process for the O element is found while the corresponding iridium element has no price changes during the charging process. At the same time, the price of manganese is different from the previous manganese-based materials. During the charging process, the manganese is not completely converted to +4 price but consists part of the +3 price. A similar phenomenon is found in the previous lithium-rich layered materials, which results from the change of O environment- caused by sliding between the laminates-around the price-changing metal. The interpretation can be supported by the phase transition from O3 to O’1 (O1).
For the first time, this study experimentally realizes the anion redox reaction of layered sodium-rich manganese-based sodium-ion battery electrode materials and provides insights for the preparation and research of layered sodium-rich materials. The research team suggests that the 5d type transition metal with strong non-metallic doping is an effective strategy to obtain layered sodium-rich materials. The researchers claim that the selection of a suitable electrolyte system should also be considered.
The research was supported by the State Key Laboratory of Solid Microstructure Physics and the Collaborative Innovation Center for Artificial Microstructure Science and Technology. It was also supported by the National Key R&D Program, the National Natural Science Foundation of China (the Youth and Youth Project) and the Jiangsu Provincial Natural Science Foundation. Special thanks to Prof. Peng Wang for his support of the transmission electron microscope characterization, and the National Synchrotron Radiation Laboratory of the University of Science and Technology of China for the help of X-ray absorption spectrum test. Associate Researcher Xiaoyu Zhang is the first author of the article, and the corresponding authors are Associate Researcher Xiaoyu Zhang, Associate Prof. Shaohua Guo and Prof. Haoshen Zhou.
(Manuscript by Xiaoyu Zhang)

