Recently, Prof. Xuebin Wang's research group has made new progress in the field of electrocatalytic water decomposition. The research group has loaded NiFeP ultra-thin nanosheets on a new type of three-dimensional ribbed graphene to obtain a self-supporting three-dimensional water decomposition electrode. Related work entitled "Monolithic electrode integrated of ultrathin NiFeP on 3D strutted graphene for bifunctionally efficient overall water splitting" was published on Nano Energy (Nano Energy, 2019, 58, 870).
Energy is an important material foundation for the survival and development of human society. The large-scale use of traditional fossil energy with limited reserves brings up problems such as environmental pollution and the greenhouse effect, which severely impedes the sustainable development of human society. As a clean secondary energy source, hydrogen has a high mass-energy ratio, and its combustion product is non-polluting. It has been regarded as an ideal energy carrier. Hydrogen production by electrocatalytic water decomposition is a very promising way. However, electrocatalytic decomposition of water confronts slow reaction kinetics of OER and HER and high overpotential. Moreover, most of the catalysts work under acidic or alkaline conditions, increasing energy consumption and production costs. Last year, the research group developed a CoO/Co4N heterostructure electrocatalyst, which achieved electrocatalytic high-efficiency total decomposition of water in a neutral system, to solve the problem of catalyst working conditions (acidic or basic). Related work "Modified Co4N as a heterostructured electrocatalyst for highly efficient overall water splitting in neutral media" was published in the Journal of Materials Chemistry A (Journal of Materials Chemistry A, 2018, 6, 24767.).
To further reduce the overpotential of electrocatalytic OER and HER and improve the reaction kinetics, Prof. Xuebin Wang's research group develops an ultra-light three-dimensional self-supporting electrode NiFeP/SG that is directly used as a reaction electrode for high-efficiency electrocatalytic oxygen evolution, hydrogen evolution reaction and complete water decomposition, based on the early research studies (Nature Communications, 2013, 4, 2905; Nano Energy, 2015, 16, 81; Bulletin of the Chemical Society of Japan, 2018, 92, 245).
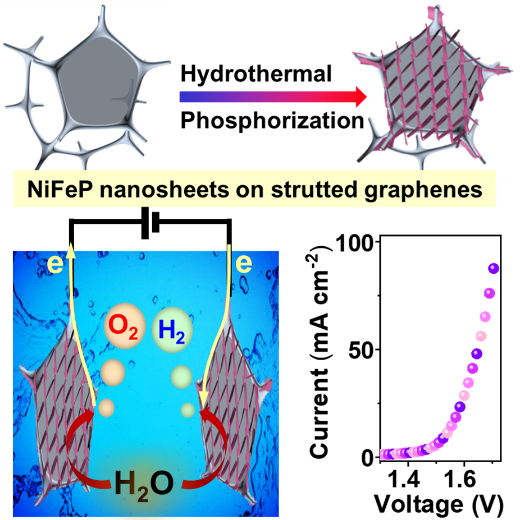
Figure 1 Preparation process of 3D NiFeP/SG electrode and its application in the electrocatalytic total decomposition of water
Due to its large specific surface area, porous structure, high electrical conductivity, and excellent stability, 3D SG is firstly used as a substrate to obtain a 3D NiFeP/ self-supporting three-dimensional water decomposition electrode by growing ultra-thin NiFeP nanosheets directly on the surface of SG. The study finds that the introduction of iron effectively adjusts the electronic structure of Ni2P, making the center of its d-band float, resulting in a decrease in the adsorption energy of the intermediate, lowering the reaction barrier, and improving the electrocatalytic activity. Moreover, the ultra-thin NiFeP nanosheet structure, the porous structure of SG and the direct growth of NiFeP on SG further enhance the catalytic activity of NiFeP/SG. When NiFeP/SG is directly used as a catalytic electrode at a current density of 10 mA cm-2, OER and HER only require overpotentials of 218 and 115 mV respectively, and the voltage of fully decomposed water is only 1.54 V. The RRDE technology proves that the OER and HER processes of 3D NiFeP/SG have high Faraday efficiency. Comparing with NiFeP/NF and NiFeP/Ti, 3D NiFeP/SG has smaller impedance and greater mass activity.
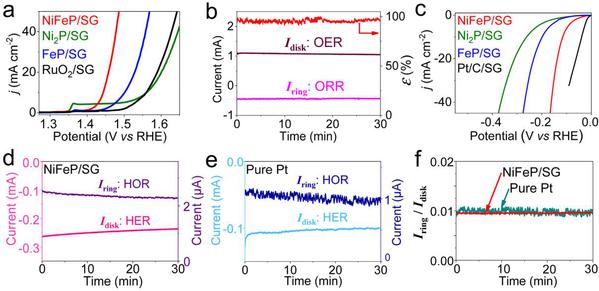
Figure 2 (a) OER polarization curve of NiFeP/SG; (b) OER faraday efficiency of NiFeP/SG sample tested by RRDE technology; (c) HER polarization curve of NiFeP/SG; (d-f) HER Faraday efficiency of NiFeP/ SG samples tested by RRDE technology
The graduate candidate Ruiqing Li at the College of Engineering and Applied Sciences is the first author of the article, and Prof. Xuebin Wang is the corresponding author. Cooperating institutions include the City University of Hong Kong and the National Institute of Materials Research in Japan. Special thanks to Prof. Yongle Li at Shanghai University and others, Prof. Libo Gao at Nanjing University and Researcher Jie Xu for their support. The research was supported by the National Natural Science Foundation of China and youth projects, the special fund for basic scientific research business expenses of central universities, Jiangsu Province's superior disciplines, and Jiangsu Natural Fund.
Related articles:
Nano Energy, 2019, 58, 870-876.
https://linkinghub.elsevier.com/retrieve/pii/S2211285519301363
J. Mater. Chem. A, 2018, 6, 24767-24772
https://pubs.rsc.org/en/content/articlehtml/2018/ta/c8ta08519f

