Nanozymes refer to functional nanomaterials with enzyme-like catalytic activity. Compared with natural enzymes, nanozymes have many advantages including low price, high stability and readily massive synthesis. Owning to the rapid development of nanotechnology, biotechnology, catalytic science and computational science, nanozymes have made many important breakthroughs (Chemical Society Reviews, 2019, 48, 1004-1076). Transition metal oxide nanomaterials are first found and studied materials that have peroxidase activity. However, the catalytic mechanism and structure-activity relationship of transition metal oxide mimicking peroxidase are not clear, which results in heavy experimental work and the lack of rational design concepts in terms of designing the metal oxide nanozymes.
In order to obtain descriptors (the indication of chemical reaction speed) that guide the design of nanozymes, researchers from Nanjing University, Jiangxi Normal University, University of Science and Technology of China and Fudan University used perovskite oxide nanomaterials as models to systematically study the effect of the electronic structure of metal oxide nanomaterials on their catalytic activity. Researchers found that there was a clear volcanic relationship between the number of eg electrons of the transition metal ion in the perovskite oxide and its catalytic activity. When the number of eg electrons is around 1, its catalytic activity is highest; when the number of eg electrons is 0 or 2, its catalytic activity is the lowest (Figure 1). Therefore, eg electron occupancy can be used as a descriptor of perovskite oxide peroxidase activity to guide the design and screening of nanozymes. However, other parameters- the number of d electrons of the transition metal ion, the center of the O 2p band, and the covalent strength of BO-have no obvious correlation with the peroxidase activity of the material, and cannot be used as descriptors to guide the design of nanozymes.
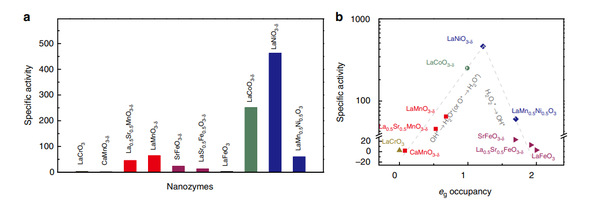
Figure 1 Evaluation of eg occupancy as an effective descriptor for catalytic activity of perovskite TMO-based peroxidase mimics
The researchers used theoretical calculations to further explain the catalytic mechanism of perovskite oxide nanozymes, and theoretically explained the reason why eg electron occupancy can be used as a peroxidase activity reaction descriptor. As shown in Figure 2, the perovskite oxide catalyzed the oxidation of TMB by hydrogen peroxide in four steps. Through further calculation, it was found that the rate-determining step is IIIb or IV when eg<1, indicating the oxidation of the substrate TMB by the adsorbed OH or O. When eg>1, the rate-determining step is II, which is the decomposition of hydrogen peroxide. The electron occupancy mainly affects the speed of the entire catalytic reaction by changing the material's adsorption ability of substrates and the rate-determining step. The nanozymes has optimized adsorption energy and can effectively promote the rate-determining step when the transition metal oxide has an eg of around 1.
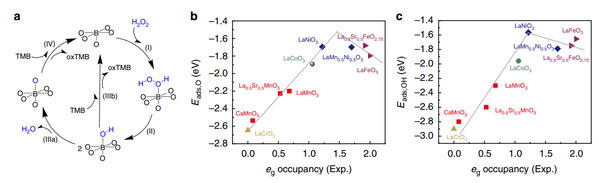
Figure 2 Computational analysis of the peroxidase-mimicking activity of ABO3 perovskite TMOs
This conclusion can be further extended to binary metal oxides with BO6 coordination. It was found that this descriptor (eg electron occupancy) is also applicable to binary metal oxides. For binary metal oxides with 0 or 2 eg electrons, the peroxidase activity is basically zero; for binary metal oxides with 1 eg electrons, the catalytic activity is high.
By occupying this descriptor with eg electrons, the perovskite oxide LaNiO3-δ with the highest activity is optimized. The researchers systematically compared the catalytic activity of LaNiO3-δ with previously reported representative nanozymes (such as Fe3O4, Cu(OH)2, GO-COOH, CuO, Co3O4, CeO2, etc.), and found that LaNiO3-δ is one to two orders of magnitude higher than the peroxidase activity of these materials whether in terms of the mass activity or the specific surface area normalized activity.
On the one hand, this work obtains the descriptors governing the activity of transition metal oxide peroxidase, which provides a theoretical basis and guidance for the rational design of metal oxide nanozymes in the future; on the other hand, the catalytic mechanism of metal oxides nanozymes is also obtained through theoretical calculations, which provides theoretical support for the further development of nanozymes. Relevant research results, entitled "eg occupancy as an effective descriptor for the catalytic activity of perovskite oxide-based peroxidase mimics", were published in Nature Communications on February 11, 2019. After the paper was published, it was reported by Phys.org as "Design principles for peroxidase-mimicking nanozymes".
The graduate candidate Xiaoyu Wang in Prof. Hui Wei's research group at Nanjing University, and Dr. Xuejiao Gao in the research group of Prof. Xingfa Gao Jiangxi at Jiangxi Normal University equally contribute to this paper. Special thanks to Prof. Li Song and the graduate candidate Changda Wang at National Synchrotron Radiation Laboratory, University of Science and Technology of China, Prof. Yongning Wang at Fudan University, Master student Li Qin, Wen Cao and graduate candidate Shichao Lin in Wei Hui Research Group, Prof. Zhong Jin and Dr. Guoyin Zhu at School of Chemistry and Chemical Engineering, Nanjing University, Prof. Kang Wang, Prof. Peng Wang, Prof. Huigang Zhang and graduate candidate Liqi Zhou at College of Engineering and Applied Science, Nanjing University. This work was supported by National Natural Science Foundation of China (21874067, 21722503, and 11874199), 973 Program (2015CB659400 and 2015CB654901), PAPD program, Shuangchuang Program of Jiangsu Province, Open Funds of the State Key Laboratory of Analytical Chemistry for Life Science (SKLACLS1704), Open Funds of the State Key Laboratory of Coordination Chemistry (SKLCC1819), Fundamental Research Funds for the Central Universities (021314380103), and Thousand Talents Program for Young Researchers.
Relative links:
1) https://www.nature.com/articles/s41467-019-08657-5.pdf
2) https://phys.org/news/2019-02-principles-peroxidase-mimicking-nanozymes.html
3) https://pubs.rsc.org/en/content/articlepdf/2019/cs/c8cs00457a

